Properties of alcohols – alcohols are polar due to the electronegative hydroxyl group(-OH) which pulls the electrons away from the carbon atom in the C-OH bond.
The electronegative oxygen in a polar hydroxyl group draws electron density away from the hydrogen in the group, giving the hydrogen a slightly positive charge. This positive charge attracts lone pairs of electrons from oxygen on neighboring molecules to form hydrogen bonds. This gives alcohols certain properties:
- when alcohols are added to water, hydrogen bonds are formed between the -OH and H2O which makes small alcohols very soluble in water. Larger alcohols are less soluble in water because more of the molecule is the non-polar chain so there is less attraction to polar H2O molecules, although they are still soluble.
- Alcohols also form hydrogen bonds with each other due to their polarity. Hydrogen bonds are the strongest intermolecular force so alcohols have a relatively low volatility compared with similar non polar compounds such as alkanes.
Classification of alcohols
Alcohols can be classified as primary, secondary or tertiary. The classification depends on how many alkyl groups (R) the carbon which the -OH is bonded to.
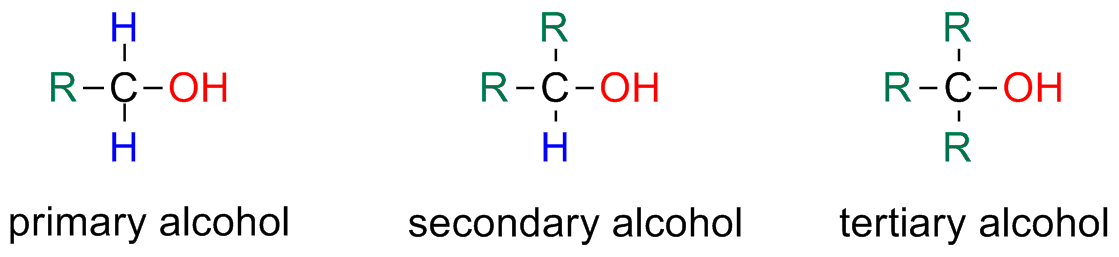
Primary – C is bonded to one alkyl group
Secondary – C is bonded to two alkyl groups
Tertiary – C is bonded with three alkyl groups
Reactions of alcohols: oxidation
Oxidation by combustion of alcohols – when alcohols are burnt, they are oxidised fully to form carbon dioxide and water.
C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(g
Oxidation of alcohols by an oxidising agent (e.g. K2Cr2O7/H2SO4):
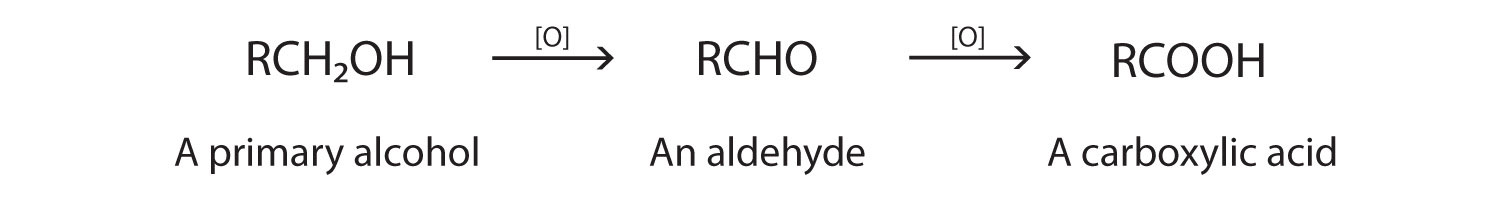
- Primary alcohols make aldehydes (distillation) and carboxylic acid (reflux)
- Secondary alcohols make ketones (reflux)
- Tertiary alcohols cannot be oxidised using an oxidising agent
Primary alcohols – forming aldehydes
To form an aldehyde, the primary alcohol must be distilled so that the aldehyde is removed as soon as it is formed and it only oxidises once.

If the primary alcohol is oxidised fully it will form a carboxylic acid. To do this the primary alcohol must be refluxed so that it oxidises twice.
Oxidising secondary alcohols – forming ketones.
Refluxing a secondary alcohol in the presence of an oxidising agent (e.g. K2Cr2O7/H2SO4) will form a ketone.
![]()

Tertiary alcohol do not oxidise in these conditions (distillation or reflux) because there is no H bonded to the carbon for the [O] to remove.
Reactions of alcohols
Elimination reaction to form alkenes –
When mixed with a concentrated acid catalyst (e.g. H2SO4 or H3PO4) and heated, an alcohol will turn into an alkene. This is an example of dehydration.
E.g. dehydration of butan-2-ol
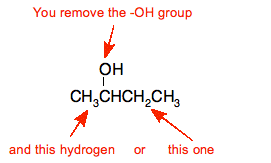
That leads to these products
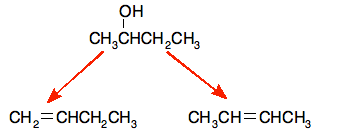
The products are but-1-ene and but-2-ene. The but-2-ene can have both an E and a Z isomer due to the stereoisomerism.
Reactions of alcohols
Substitution with halide ions to form haloalkanes.
- Alcohols react with compounds containing halide ions (such as from NaBr) in a substitution reaction.
- The hydroxyl group is replaced with the halide ion to form the haloalkane.
- The reaction can happen at room temperature but requires an acid catalyst (e.g H2SO4 or H3PO4) in order to happen.
