Hydrogen atoms and the electrons they possess are a valuable source of energy. These hydrogen atoms are carried by coenzymes NAD and FAD into the next stage of the process: the electron transport chain.
This is the mechanism by which the energy of the electrons within the hydrogen atoms is converted into a form that cells can use – ATP.
The Electron Transport Chain and Mitochondria
Mitochondria are rod-shaped organelles that are found in eukaryotic cells. Each mitochondria is bounded by a smooth outer membrane and an inner one that is folded into extensions called cristae.
The inner space, or matrix, of the mitochondrion is made up of a semi-rigid material of protein, lipids and traces of DNA.
Mitochondria play an active role in respiration and the release of energy.
The Electron Transport Chain and the Synthesis of ATP
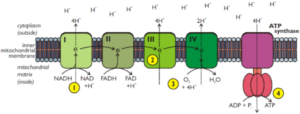
1. The hydrogen atoms produced during glycolysis and the Krebs cycle combine with the coenzymes NAD and FAD that are attached to the cristae of the mitochondria
2. The reduced NAD and FAD donate the electrons of the hydrogen atoms they are carrying to the first molecule in the electron transport chain
3. This releases the protons from the hydrogen atoms and these protons are actively transported across the inner mitochondrial membrane
4. The electrons pass along a chain of electron transport carrier molecules in a series of oxidation-reduction reactions
The electrons lose energy as they pass down the chain and some of this is used to combine ADP and inorganic phosphate to make ATP
The remaining energy is lost in the form of heat
5. The protons accumulate in the space between the two mitochondrial membranes before they diffuse back into the mitochondrial matrix through special protein channels Energy is used to pump the protons across
6. At the end of the chain the electrons combine with these protons and oxygen to form water Oxygen is therefore the final electron transport chain