Electromagnetic Radiation (EMR)
-
EMR: energy that exhibits wavelike behavior and travels thru space at the speed of light in a vacuum
- Ex: light from the sun, X-rays
- All EMR travel at speed of light
- Each form of EMR is only different in wavelength
-
Photon: tiny particle of light that acts as a carrier of energy
- Shorter wavelength = higher frequency = more energy
- So there is an inverse relationship between wavelength and frequency
Essential Formulas
- Formula:
3 Characteristics of Waves
| Wavelength |
Frequency |
Speed of light |
| ● Distance between two consecutive peaks
● Unit = Meters ■ 1m = 10⁹ nm |
● The number of waves(cycles) per second
● Unit = s⁻¹ (Hertz) |
● 2.9979 X 10⁸ |
Albert Einstein
- The intensity of light is a measure of the number of photons in a beam
- Greater intensity = more photons are available to release electrons
-
- Mass (kg) of a particle:
- v = velocity
- Can also use equation to calculate wavelength of a particle
The Dual Nature of Light: EMR can show both wave properties and particulate matter properties
- Electrons seem to move in an interference pattern (like a wave) and have the ability to carry energy and momentum when in motion (like a particle) → an electron is both a wave and particle
The Bohr Model
- Predicted that electrons orbit the nucleus at fixed radii
- Atoms absorb energy in the form of electromagnetic radiation → electrons move to higher energy lvl
- As electrons become more tightly bound, its energy becomes more negative
- As the electron is brought closer to the nucleus, energy is released from the system
- Is wrong bcuz does not take into account for sublevels (s, p, d, f), orbitals, or electron spin → electrons aren’t actually locked into orbits
The Quantum Mechanical Model of the Atom
-
Quantum Mechanical Model: specifies the probability of finding an electron in the 3D space
-
Wave function of an electron: the amplitude where an electron can stay in an atom
- Square of the wave function = the probability of the electron in the orbital
| Shell | Subshell | Orbital |
| Pathway followed by an electron around the atom’s nucleus
Given n Can hold max 32 e |
Pathway an electron moves inside the shell
Given l Maximum e- depends on type of subshell |
Most probable location to find an electron and its spatial distribution
Given m Can hold max 2 e- Energy of a orbital is determined by its value of n → all orbitals with the same value of n have the same energy (are said to be degenerate) Orbital configurations determine the shape of a molecule → determines their properties and how they behave
|
Heisenberg Uncertainty Principle
- Says that it’s impossible to know the exact motion/position of an electron as it moves around the nucleus
- Two types of electron motions in an atom
- Orbit motion around a nucleus
- Magnetic motion created by the spin on its axis
- The probability of finding the electron at a specific position is greatest the closer it is to the nucleus
Different Types of Wave Functions
| S Sublevel | P Sublevel | D Sublevel | F Sublevel |
| 1 orbital = max 2 electrons → the simplest | 3 orbitals = max 6 e-
There are 3 P orbitals because are talking about 3D space → can be one orbital on the x-axis, y-axis, and z-axis ● Each axis canhold a total of 8electrons (s=2 &p=6)→ octet rule |
5 orbitals → max 10electrons | 7 orbitals → max 14electrons |
Quantum Numbers
- Each of these orbitals is characterized by a series of numbers called quantum numbers
| Name | Symbol | Allowed Values | Notes |
| Principle quantum Number | n | 1, 2, 3, 4, … | Describes the size and energy level of an orbital + relative distance from nucleus
Is equal to the number of sublevels
n increases → orbital becomes larger = higher energy electrons |
| Angular momentum/azimuthal quantum number | 1 | 0 ≤ L ≤ n-1 | Describes the shape of an orbital
L = 0 → s orbital L = 1 → p orbital L = 2 → d orbital L = 3 → f orbital L ≤ n-1 ● Ex: n = 2
|
| Magnetic quantum number | m | L ≤ m ≤ L | Describes the orientation of the orbitalTo determine value, draw orbitals → place electron → ans will be where the last electron is
Ex: ● S sublvl has 1 orbital and l = 0→ m = 0 ● P sublvl has 3 orbitals & l = 1→ -1 ≤ m ≤1 |
| Spin quantum number | m | +½. -½ | Describes the spin of an e-→ an e- can only spin in a clockwise or counter-clockwise direction
+ ½ ( up arrow) or -½ (down arrow) Answer will be the spin of the last electron placed |
Example: 2p⁵ → find n, l , m◻, m◻
- N =2
- L =1
- m◻→ 0
- Ms → -½
Important Principles
-
Pauli Exclusion Principle: two electrons which share an orbital cannot have the same spin → have different values of m◻
-
Aufbau principle: electrons are placed in orbitals, shells, and subshells of increasing energy
-
Hund’s rule: Every orbital in a sublevel is singly occupied before any orbital is doubly occupied + All of the electrons in singly occupied orbitals have the same spin
- Because of the symmetrical distribution of electrons, orbitals in which the subshell is exactly half-filled or filled are more stable → so removing an electron from these atoms requires more energy
Radial Probability, Penetration, and Electron Repulsion
- Electrons are attracted to the nucleus at the same time as electrons repel each other.
-
Penetration: The ability of an electron to get close to the nucleus
- Penetration depends on the shell (n) and subshell (ml)
- Within same shell value (n), penetrating power follows trend in subshells (ml)
- s>p>d>f
- For different shall values and subshell (l), penetrating power follows this trend
- 1s>2s>2p>3s>3p>4s>3d>4p>5s>4d>5p>6s>4f….
- Within same shell value (n), penetrating power follows trend in subshells (ml)
- The closer an electron comes to the nucleus/more it penetrates → the stronger its attraction to the nucleus
- Penetration and shielding result in an Effective force that holds the outer electrons to the atom
- Penetration depends on the shell (n) and subshell (ml)
Electron Shielding
-
Shielding: describes the blocking of the attraction between valence electrons and nucleus by the inner-shell electrons
- Results from balance between attractive and repulsive forces
Writing electron configuration
● Electron configuration: tell us where electrons are approx
○ For D block elements, count starting from S block
● Noble gas Configuration: consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons
○ Use as shortcut → on AP exam can always use
● Ex:
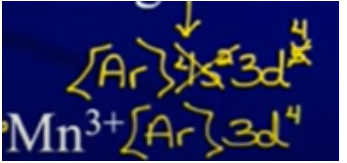
Ion Configurations
- Electrons are removed/added to the valence energy lvl first → only then can e- be removed/added from the d sublvl
- Ex:
Write out configuration for pure element first and then remove/add electrons
- Can’t just keep noble gas in brackets → have to back up to noble gas before