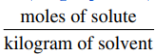- Molarity (M): A.K.A Concentration
- Ex: A solution that is 1.0 molar (written as 1.0 M) contains 1.0 mole of solute per liter of solution.
- Note: brackets around something it means the “molarity of what is inside”
- Dilution: water is added to achieve the molarity desired for a particular solution
○ Does not change the amount of moles present; ex: halve volume = double molarity
- Mass percent (weight percent):
- Mass percent (weight percent):
- Molality:
- Normality (N): Molarity x number of equivalents (definition of an equivalent depends on the reaction taking place in the solution)
○ Acid–base reaction → number of protons = equivalents
○ Oxidation–reduction reactions → number of e- in half-reaction = equivalents
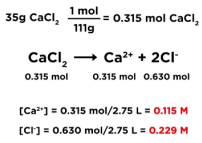
Calculating Concentration of Ions
- Write out balanced formula for dissolution reaction
- Ex: 35g CaCl2 dissolved in 2.75L
Steps in Solution Formation
- Energy absorbed to overcome solute-solute interactions (endothermic) (ΔH₁)
- Breaking ionic compound= breaking ionic bonds; breaking covalent solute = breaking interMF
- Energy absorbed to overcome solvent-solvent interactions to make room for the solute (endothermic) (ΔH₂)
- Energy released by allowing the solute and solvent to interact to form bonds and a solution (exothermic) (ΔH₃)
- Enthalpy (heat) of solution (ΔHsoln): is the sum of the ΔH values for the steps of solution formation
○ In dissolving the change in energy equals the sum of the energies required to separate solute particles from one another and solvent particles from one another minus the energy released when attractions between solute particles and solvent particles form